Why Are Chemical Bonds Important
Introduction to Chemical Bonding
- Page ID
- 1644
Chemical bonding is one of the nigh basic fundamentals of chemical science that explains other concepts such every bit molecules and reactions. Without it, scientists wouldn't be able to explain why atoms are attracted to each other or how products are formed after a chemical reaction has taken place. To understand the concept of bonding, ane must first know the nuts behind atomic structure.
Introduction
A common atom contains a nucleus composed of protons and neutrons, with electrons in certain energy levels revolving effectually the nucleus. In this section, the main focus will be on these electrons. Elements are distinguishable from each other due to their "electron deject," or the area where electrons move around the nucleus of an atom. Because each element has a singled-out electron deject, this determines their chemical properties every bit well every bit the extent of their reactivity (i.e. noble gases are inert/not reactive while alkaline metals are highly reactive). In chemical bonding, but valence electrons, electrons located in the orbitals of the outermost free energy level (valence shell) of an element, are involved.
Lewis Diagrams
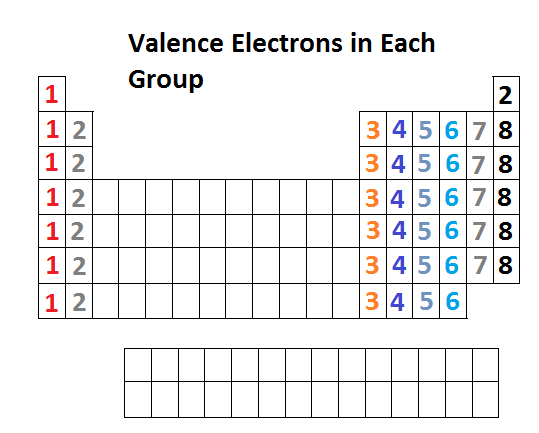
Lewis diagrams are graphical representations of elements and their valence electrons. Valance electrons are the electrons that course the outermost shell of an atom. In a Lewis diagram of an chemical element, the symbol of the element is written in the center and the valence electrons are drawn effectually it as dots. The position of the valence electrons drawn is unimportant. Yet, the full general convention is to outset from 12o'clock position and go clockwise direction to 3 o'clock, vi o'clock, ix o'clock, and dorsum to 12 o'clock positions respectively. Generally the Roman numeral of the group corresponds with the number of valance electrons of the element.
Below is the periodic table representation of the number of valance electrons. The alkali metals of Group IA take i valance electron, the alkaline-earth metals of Group IIA take 2 valance electrons, Group IIIA has 3 valance electrons, and then on. The nonindicated transition metals, lanthanoids, and actinoids are more hard in terms of distinguishing the number of valance electrons they have; however, this section only introduces bonding, hence they will not be covered in this unit.
Lewis diagrams for Molecular Compounds/Ions
To depict the lewis diagrams for molecular compounds or ions, follow these steps beneath (we will be using H2O as an example to follow):
1) Count the number of valance electrons of the molecular chemical compound or ion. Remember, if in that location are ii or more than of the same element, so you have to double or multiply by withal many atoms there are of the number of valance electrons. Follow the roman numeral group number to see the corresponding number of valance electrons there are for that element.
Valance electrons:
Oxygen (O)--Group VIA: therefore, there are 6 valance electrons
Hydrogen (H)--Grouping IA: therefore, at that place is one valance electron
NOTE: In that location are Two hydrogen atoms, so multiply 1 valance electron X two atoms
Total: 6 + 2 = viii valance electrons
two) If the molecule in question is an ion, recollect to add together or subract the corresponding number of electrons to the total from pace one.
For ions, if the ion has a negative charge (anion), add the corresponding number of electrons to the total number of electrons (i.e. if NO3 - has a negative accuse of i-, then you add together ane extra electron to the total; 5 + 3(half dozen)= 23 +i = 24 full electrons). A - sign hateful the molecule has an overall negative charge, and so information technology must have this extra electron. This is because anions have a higher electron analogousness (tendency to gain electrons). Nigh anions are composed of nonmetals, which have high electronegativity.
If the ion has a positive charge (cation), decrease the corresponding number of electrons to the total number of electrons (i.east. HthreeO+ has a positive charge of i+, so you subtract 1 extra electron to the full; half dozen + i(three) = 9 - 1 = 8 total electrons). A + sign means the molecule has an overall positive charge, and then it must be missing one electron. Cations are positive and have weaker electron affinity. They are by and large equanimous of metals; their atomic radii are larger than the nonmetals. This consequently means that shielding is increased, and electrons have less tendency to be attracted to the "shielded" nucleus.
From our example, h2o is a neutral molecule, therefore no electrons demand to be added or subtracted from the total.
3) Write out the symbols of the elements, making sure all atoms are deemed for (i.e. H2O, write out O and 2 H's on either side of the oxygen). Start by adding unmarried bonds (ane pair of electrons) to all possible atoms while making sure they follow the octet rule (with the exceptions of the duet dominion and other elements mentioned above).
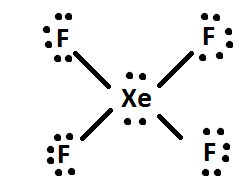
iv) If at that place are any leftover electrons, then add them to the central cantlet of the molecule (i.eastward. XeF4 has iv actress electrons after being distributed, so the 4 extra electrons are given to Xe: like and then. Finally, rearrange the electron pairs into double or triple bonds if possible.
Octet Dominion
Most elements follow the octet rule in chemical bonding, which ways that an element should accept contact to eight valence electrons in a bond or exactly fill up its valence vanquish. Having 8 electrons total ensures that the cantlet is stable. This is the reason why noble gases, a valence electron crush of viii electrons, are chemically inert; they are already stable and tend to non need the transfer of electrons when bonding with some other atom in order to be stable. On the other manus, alkali metals accept a valance electron shell of i electron. Since they want to consummate the octet rule they oftentimes simply lose one electron. This makes them quite reactive because they tin hands donate this electron to other elements. This explains the highly reactive properties of the Group IA elements.
Some elements that are exceptions to the octet dominion include Aluminum(Al), Phosphorus(P), Sulfur(S), and Xenon(Xe).
Hydrogen(H) and Helium(He) follow the duet rule since their valence beat out but allows two electrons. There are no exceptions to the duet dominion; hydrogen and helium will always hold a maximum of ii electrons.
Ionic Bonding
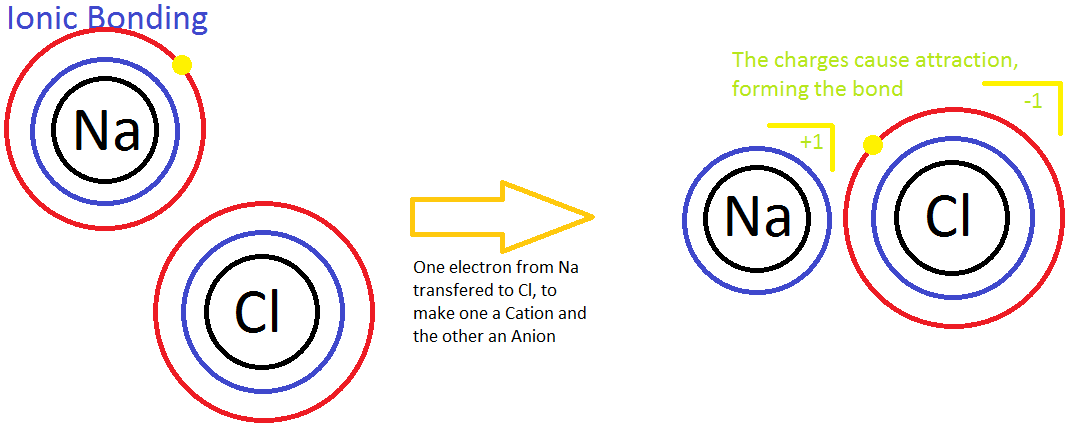
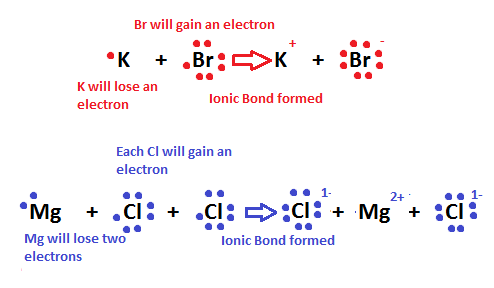
Ionic bonding is the process of non sharing electrons between ii atoms. It occurs betwixt a nonmetal and a metallic. Ionic bonding is besides known every bit the process in which electrons are "transferred" to 1 another considering the 2 atoms have dissimilar levels of electron analogousness. In the motion picture below, a sodium (Na) ion and a chlorine (Cl) ion are being combined through ionic bonding. Na+ has less electronegativity due to a big atomic radius and substantially does not desire the electron it has. This will easily allow the more electronegative chlorine atom to gain the electron to complete its 3rd energy level. Throughout this process, the transfer of the electron releases energy to the temper.

Some other instance of ionic bonding is the crystal lattice construction shown above. The ions are arranged in such a fashion that shows unifomity and stablity; a physical characteristic in crystals and solids. Moreover, in a concept chosen "the sea of electrons," it is seen that the molecular structure of metals is composed of stabilized positive ions (cations) and "complimentary-flowing" electrons that weave in-between the cations. This attributes to the metallic property of conductivity; the flowing electrons allow the electric current to pass through them. In addition, this explains why strong electrolytes are expert conductors. Ionic bonds are easily broken by water considering the polarity of the h2o molecules shield the anions from attracting the cations. Therefore, the ionic compounds dissociate easily in water, and the metallic backdrop of the compound allow conductivity of the solution.
Covalent Bonding
Covalent bonding is the process of sharing of electrons betwixt 2 atoms. The bonds are typically between a nonmetal and a nonmetal. Since their electronegativities are all within the high range, the electrons are attracted and pulled by both atom's nuceli. In the case of 2 identical atoms that are bonded to each other (likewise known as a nonpolar bond, explained later below), they both emit the same forcefulness of pull on the electrons, thus in that location is equal attraction betwixt the 2 atoms (i.due east. oxygen gas, or Oii, take an equal distribution of electron analogousness. This makes covalent bonds harder to pause.
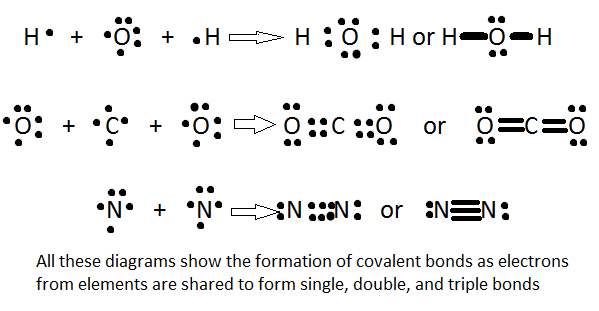
There are 3 types of covalent bonds: single, double, and triple bonds. A unmarried bond is composed of 2 bonded electrons. Naturally, a double bail has 4 electrons, and a triple bail has 6 bonded electrons. Because a triple bond volition take more force in electron affinity than a single bond, the attraction to the positively charged nucleus is increased, meaning that the distance from the nucleus to the electrons is less. Just put, the more bonds or the greater the bond strength, the shorter the bond length volition be. In other words:
Bond length: triple bond < double bond < single bond
Polar Covalent Bonding
Polar covalent bonding is the process of unequal sharing of electrons. Information technology is considered the middle basis between ionic bonding and covalent bonding. It happens due to the differing electronegativity values of the two atoms. Because of this, the more than electronegative atom will attract and have a stronger pulling force on the electrons. Thus, the electrons will spend more time effectually this atom.
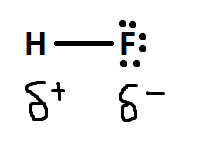
The symbols above betoken that on the flourine side it is slightly negitive and the hydrogen side is slightly positive.
Polar and Non-polar molecules
Polarity is the competing forces between 2 atoms for the electrons. It is also known every bit the polar covalent bond. A molecule is polar when the electrons are attracted to a more electronegative atom due to its greater electron analogousness. A nonpolar molecule is a bail between two identical atoms. They are the ideal example of a covalent bond. Some examples are nitrogen gas (Ntwo), oxygen gas (O2), and hydrogen gas (H2).
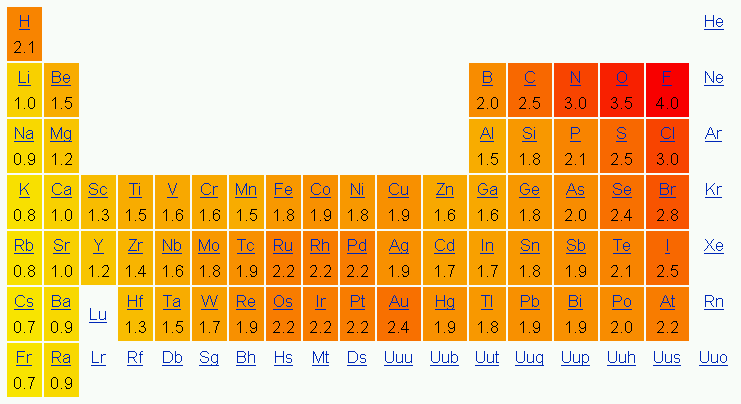
One mode to figure out what type of bond a molecule has is by determining the difference of the electronegativity values of the molecules.
If the divergence is between 0.0-0.3, then the molecule has a not-polar bond.
If the deviation is between 0.3-i.vii, then the molecule has a polar bail.
If the difference is i.7 or more than, then the molecule has an ionic bond.
Why Are Chemical Bonds Important,
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_%28Inorganic_Chemistry%29/Chemical_Compounds/Introduction_to_Chemical_Bonding
Posted by: christensenevisold.blogspot.com


0 Response to "Why Are Chemical Bonds Important"
Post a Comment